
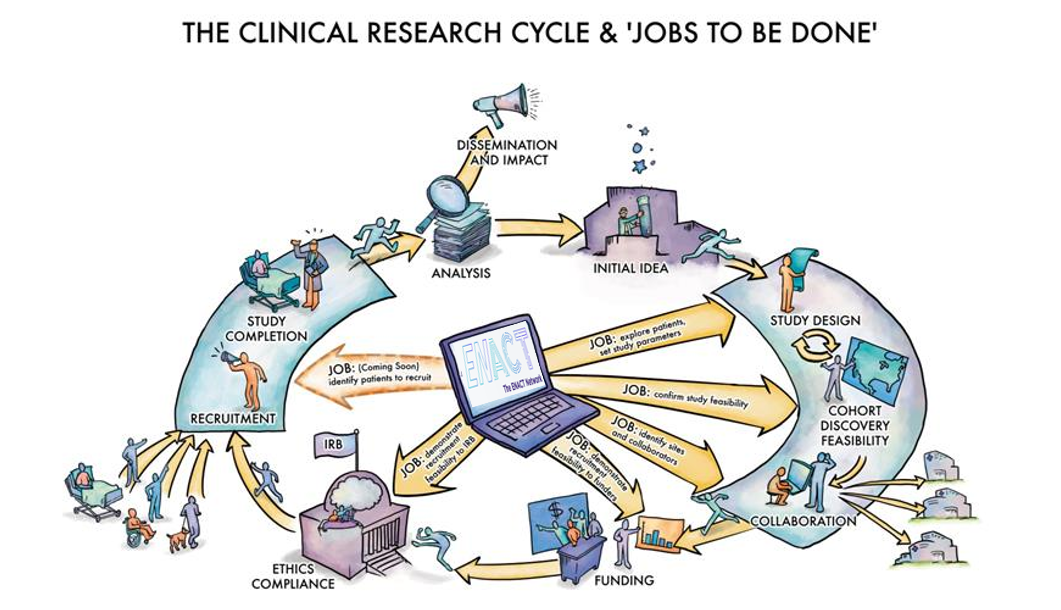
The ENACT Network ("Evolve to Next-Gen ACT," a progression from the previous Accrual to Clinical Trials Network) is an IT platform that allows investigators to query de-identified EHR data across the CTSA Consortium under a data governance agreement that allows researchers to explore and validate feasibility
for clinical studies by themselves from their desktop, in minutes. ENACT helps clinical investigators conduct cohort discovery before a trial starts, to establish feasibility of a clinical protocol for grant applications, IRB submission, etc. ENACT also
helps investigators identify additional sites for a clinical trial. By allowing investigators to thoroughly explore patient cohorts and potential sites before finalizing their clinical protocols, ENACT increases the odds of successfully completing clinical
trial recruitment. ENACT was developed collaboratively by members of NCATS’ Clinical and Translational Science Award (CTSA) Consortium, with funding from the NIH National Center for Advancing Translational Sciences.
Using ENACT, clinical investigators can:
- Conduct early-stage cohort discovery and explore patient populations
- Test and refine study eligibility criteria in real-time
- Identify potential partner sites for multi-site studies
- Demonstrate study feasibility in funding proposals and IRB submissions
Who can use it? (Open to anyone at UTMB.)
How do I get access? You can submit a request for services at https://utmb.us/3ve.
Find your Trusted Requester here!
How do I cite ENACT in my grant or publication?
Publications based on research using the ENACT Network must cite the NCATS ENACT grant: "This project was supported by the National Institutes of Health through grant 1U24TR004111-01." Publications should also cite the appropriate CTSA Hub grant numbers from participating institutions.
Any Intellectual Property derived from use of the ENACT Network must cite the NCATS ENACT grant: "This project was supported by the National Institutes of Health through grant 1U24TR004111-01."
Publications in which data source partners (hospitals) are to be identified by name will be reviewed for use of name only by each identified hospital prior to submission of a manuscript. At no time will specific participating data source partners be named unless explicitly approved by the data partner. Such approval must be requested and received in writing between the requestor and the Senior Vice President of Research, the Chief Information Officer, or their respective designee. Any entity (e.g., hospital) that does not agree to be identified by name as a data source will be instead identified as a "CTSA-affiliated hospital."
To learn more or use ENACT, visit the ENACT Network.
Get the ENACT Network flyer here!
Contact: ITS.RIS@utmb.edu.